Platform Technology
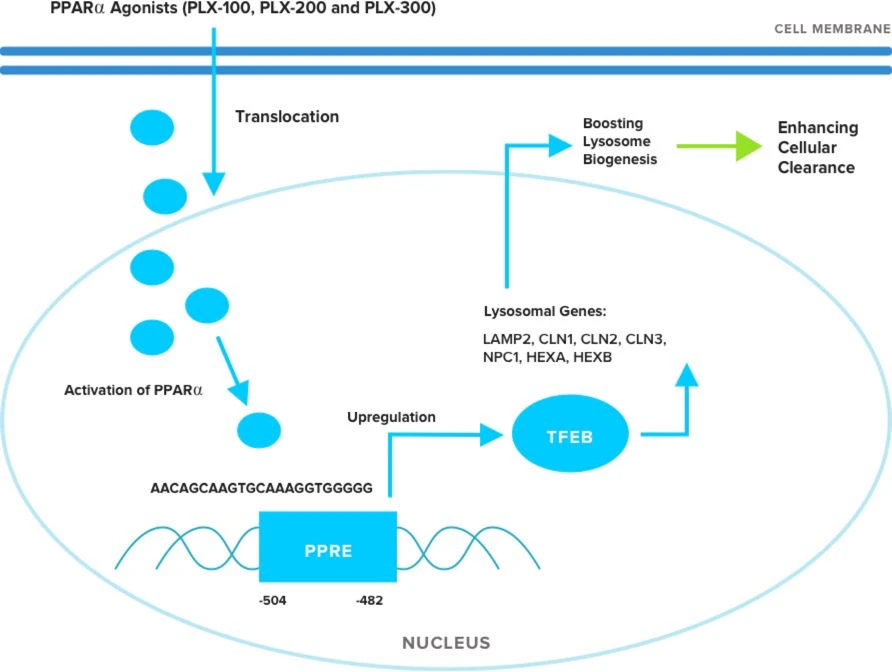
Lysosomes are central to eliminating tissue toxic wastes by either the autophagic, endocytic, or phagocytic pathway. In addition, lysosomes are an integral part of cellular signaling pathways. Transcription factor EB (TFEB), a master regulator of lysosomal biogenesis, coordinates the functionality and activity of lysosomes in the cell. Thus, boosting lysosomal biogenesis through TFEB upregulation is an attractive therapeutic intervention for lysosomal storage disorders in which toxic aggregates are responsible for the disease pathogenesis.
We have demonstrated that our drug candidates, PLX-100, -200, and -300, are PPARα agonists, and thus can upregulate TFEB expression in brain cells. Our drug candidates also upregulate TFEB expression when tested in combination with all trans retinoic acid (ATRA), an agonist of RXRα by the formation of a PPARα/ RXRα heterodimer.
Polaryx’s® platform technology can be applied to treat lysosomal storage disorders in which cellular toxic wastes are responsible for the disease pathogenesis.
Targeted Indications
Neuronal Ceroid Lipofuscinosis (NCL)
Neuronal ceroid lipofuscinosis (NCL), also known as Batten disease, is a group of rare inherited lysosomal storage disorders affecting 1-4 children/100,000 live births. NCL leads to premature death. The major characteristic of NCL is an excessive accumulation of auto-fluorescent lipofuscin in both neuronal and other cells in the patients.
Because NCL starts with seizures and/or vision failure followed by progressive motor dysfunction and cognitive decline, it is often misdiagnosed as one of the other neurological disorders.
NCLs are inherited in an autosomal recessive pattern, and their frequencies vary based on the genetic mutations. Over 400 different mutations have been found in 14 different genes so far. Most of the proteins encoded by them are soluble enzymes (CLN1/PPT1, CLN2/TPP1, CLN10/CTSD, SLN13/CTSF) in the lysosome, soluble lysosomal protein (CLN5), and transmembrane proteins (CLN3, CLN6, CLN7/MFSD8, CLN8, CLN12/ATP132A). Among them, CLN6 and CLN8 localize in the endoplasmic reticulum, while CLN4/DNAJC5 and CLN14/KCTD7 are cytoplasmic and associate with cell membranes. However, their physiological substrates and functions are unknown. Such multiple responsible genes and various gene mutations make therapeutic interventions, including gene therapy, very challenging.
Niemann Pick Disease Types A and B
Acid sphingomyelinase deficiency, including Niemann Pick Disease types A and B, is caused by genetic mutations in acidic sphingomyelinase (ASMase), the lysosomal enzyme that degrades sphingomyelin. Deficiencies in ASMase or its activity result in a reduced break-down and increased levels of intracellular sphingomyelin leading to cellular dysfunction and cell death. Over time, the cell loss in the organs of children with NPD impairs the function of the brain, lungs, spleen, muscle, and liver with a wide range of symptoms that vary in severity. Especially, sphingomyelin accumulation disrupts the membranes of various subcellular organelles within neurons and glial cells, leading to anomalies in signaling, polarization, calcium homeostasis, synaptic plasticity, and myelin production. These pathologies lead to motor and muscle dysfunction., hearing and vision loss, and early death.
Krabbe Disease
Krabbe disease or globoid-cell leukodystrophy (GLD) is a rare inherited lysosomal storage disorder that leads to the premature death of young children. This autosomal recessive disease, which is caused by a deficiency of galactosylceramidase (GALC), leads to an altered catabolism of galactosylceramide and an increase in the level of the toxic glycolipid, psychosine. Several studies have shown that psychosine is very toxic for oligodendrocytes, and rapid demyelination is a key pathological feature of GLD. Clinically, it is characterized by irritability, spasticity, seizures, and death by 2 years of age. Despite many studies, no effective drug is currently available to halt and/or delay the progression of GLD.
GM2 Gangliosidosis
GM2 gangliosidosis, also known as Tay-Sachs and Sandhoff diseases, are ultra-rare and fatal pediatric neurodegenerative disorders caused by defects in Hexosaminidase A (HEXA) and Hexosaminidase B (HEXB), key enzymes in the lysosome, respectively. These genetic defects lead to abnormal accumulation of gangliosides, resulting in severe progressive neurodegeneration, seizures, loss of mobility, hearing, and vision, and early death. There is no cure for these diseases and the only treatment is supportive care.
Batten disease presents as seizures and / or vision failure followed by progressive motor dysfunction and cognitive decline.